The challenge
Digital technologies have become integral to all sectors, and none more so than the healthcare industry.
Companies in the healthcare industry are investing significantly in the conception, development, manufacturing, sale and distribution of “HealthTech”. It is important to protect and ensure a return on this investment through a strategic use of the available IP and trade secrets laws. In particular, it is important to take a holistic approach to the use of IP and trade secrets laws to protect and ensure a return on your investment in HealthTech innovations. as each stream plays its own role in protecting the overall technology. This involves complex IP and trade secret considerations, made even more complex by the evolving law on whether patent protection is available for computer-implemented technologies. Today, we take a brief look at some emerging HealthTech. We then turn to how current IP and trade secrets laws can be used to protect these innovations and some of the key issues that need to be considered when determining which IP rights to deploy as part of your IP protection strategy.
Tomorrow’s highlights
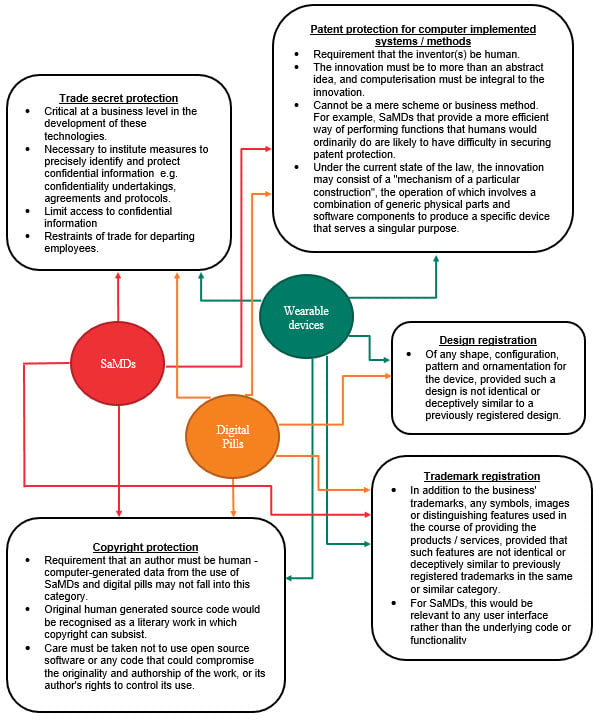
Three exemplars of HealthTech innovations are SaMDs, digital pills and wearable health devices:
(1) Software as a Medical Device (SaMDs), is software that is intended to be used for one or more medical purposes without being part of a hardware medical device. SaMDs have recently become a regulatory focus for Australia’s Therapeutic Goods Administration (TGA) – in June 2020, the TGA released its guidance on the regulation of SaMD in Australia. SaMD refers to software that fits within the definition of a medical device under section 41BD of the Therapeutic Goods Act 1989 (Cth), examples of which include:
- smart phone apps that calculate insulin doses based on a patient’s blood glucose levels;
- X-ray image-processing software; and
- software that uses information about a patient to make a diagnosis.
The following however are not SaMDs:
- health and lifestyle apps; or
- medical device software that is an integral part of a physical device (for example embedded software or firmware in a cardiac pacemaker).
(2) Digital pills, which (for now) constitute ingestible sensors that send patient information to external devices.
(3) Wearable health devices, which are now commonplace in today’s society. Examples include heart rate monitors and smartwatches that collect personal health information that can be analysed and shared.
Today’s laws
What’s next?
IP protection for HealthTech is a hot topic. The next couple of years will likely give us further TGA regulations and court judgments that can broaden our understanding of the issues that will affect these emerging technologies. For now, it’s a case of wait and watch.



